Abstract
The purpose of this experiment was to determine whether texture plays a significant role in determining the efficacy of electroosmosis in effecting the removal of organic, water-soluble contaminants from soil. Soil samples were collected which provided a wide assortment of attributes for a basis of comparison and analysis. The soil samples were oven-dried to assure the equal saturation of all the soil samples by equal concentrations of tannic acid.
A test chamber was constructed from a fluorescent tube protective cover and the end contacts of a fluorescent lamp. Three holes equidistant from one another were drilled in the tube in order to allow a measurement of tannic acid at regular intervals along the chamber. The test solution of tannic acid was 100 mg/L.
Each sample was saturated with tannic acid solution to the point that all pores were occupied. The test chamber was then filled with the saturated soil and the end caps sealed. An initial sample was taken from the test chamber in order to substantiate the initial concentration. A direct current of eight volts was then applied to the test chamber across the end contacts by means of a power supply. Every two hours for a total of six hours water samples were taken from each of the testing ports by means of pipettes. Each water sample was tested for tannic acid concentration.
The data collected show a tentative relationship of texture to electroosmotic efficacy and a distinct correlation between pore space and electroosmotic efficacy.
INTRODUCTION
Electroosmosis
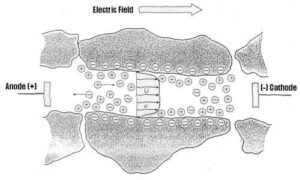
Electroosmosis is a term applied to the process in which a liquid containing ions moves relative to a charged stationary surface . The phenomenon of electroosmosis has been applied in numerous ways, including as a means of dewatering soils for construction purposes and dewatering mine tailings and waste sludges. Recently, electroosmosis has been investigated as a potentially valuable in situ (i.e., on-site) method of purging contaminants from soil. In this respect, electroosmosis operates by causing an effluent (water) to move relative to a stationary charged surface, which is in this case the collective capillary pores of the soil. Another element of electroosmosis is the migration of the contaminant particles themselves relative to the liquid and the soil profile. This specific element is sometimes called electromigration and viewed separately from electroosmosis, although the two processes cannot be separated from one another due to the nature of their operation.
Electroosmosis research has been largely limited to the laboratory, and only in certain locations have large-scale experiments been conducted in a natural setting. The majority of [published] laboratory experiments concerning the remediary potential of electroosmosis has been limited to tests on kaolinite clay samples; one of the main goals of this project was to extend tests of electroosmosis past uniform samples to tests on soils of various texture. Although electroosmosis (in theory) should be more effective in soils with a lower hydraulic conductivity (i.e., soils with a greater percentage of micropores and that are more difficult to move water through by means of a pressure-driven device), this research sought to determine how great an effect texture has on the efficacy of electroosmosis by experimental means.
Soil Contamination and Remediation
As the technological level of our society continues to increase rapidly, the variety of new kinds of chemical wastes that we produce has also increased. As these chemical wastes continue to multiply and diversify, it is becoming more and more important that our methods of minimizing the impact of these wastes improve at the same rate. Although one important step in this process is the minimizing of the production of such wastes, some chemical by-products are inevitably produced and some inevitably escape into the environment. Most cases of direct contamination of the environment by chemicals include contamination of the soil. To worsen matters, soil is, in one way or another, of great importance to every plant and animal on this planet. When contaminated, soil can also serve as a means of spreading contamination throughout the environment. It is for these reasons that it is important that effective methods of remediating contaminated soil be developed.
An important aspect of the process of soil remediation is the ability to correctly recognize the dominant mechanism for the removal of the contaminants-that is, the method of soil remediation that can most effectively (both environmentally and economically) be used. In making this decision, it is necessary to take into account the nature of the contaminant itself, the characteristics of the soil, and the characteristics of the various methods of remediation. The main goal of this research was to determine whether or not texture should be a factor in considering the use of electroosmosis as a method of soil remediation.
Optimal remediation of contaminated soil also demands that unnatural (or extreme quantities of natural toxins) be removed effectively with minimal processing or alteration of the natural chemistry and morphology of the soil and surrounding environment. The investigation of new technologies and methods of remediation is necessary to attain these goals. Additionally, demonstration of the relationships of efficacy to the variables present in both soil and the contaminant will be required.
Soil Contaminants
In this research the contaminant chosen was tannic acid mainly because it is structurally similar to a widespread group of organic, water-soluble contaminants known as the aromatics. Organic contaminants are more difficult to remove by electrokinetic means than metallic compounds, namely due to the fact that they have a low polarity. However, electroosmosis has still been demonstrated to be a potentially valuable method of effecting the removal of such aromatics due to the migration coefficient of the effluent.
Soil Contaminants: The Aromatics
Aromatics are some of the most important organic chemicals both in industrial processes and to the consumer. The principal members of this group are toluene, benzene, and xylene, which are liquid hydrocarbons that yield a number of derivatives and combine with other organic chemicals to produce compounds such as styrene (which is a product of benzene, a member of the aromatics, and ethylene). One of the compounds that aromatics makes possible is motor gasoline. Benzene is used primarily to produce intermediate chemicals such as styrene, cumene, and cyclohexane, which are then used in industrial processes to produce polystyrene, phenol, and nylon. Mixed xylenes are utilized in many industrial processes as well, both as solvents and as chemical intermediates to produce a number of plastics, resins, and synthetic fibers. Additional products of the aromatics are synthetic rubber, detergents, latex, and polyurethanes. It is this wide variety of uses and applications that makes the aromatics particularly ubiquitous, both in industry and household, and therefore, common contaminants of soil. This fact, coupled with the potential hazard of these chemicals, makes it even more important that we develop effective methods of dealing with such chemicals.
Advantages of Electroosmosis over Conventional Methods of Soil Remediation
In situ methods of soil remediation such as electroosmosis hold obvious advantages over more conventional methods which can involve physically removing or greatly disturbing a large portion of the contaminated soil. Although the addition of pure water to an ecosystem can in itself be disturbing in some cases, electroosmosis is much less destructive to the natural environment than conventional methods which use pressure-driven water flow to remove contaminants. Because electroosmosis operates on a particle level rather than on a macro level, it has virtually no impact on the structure of the soil.
Many contaminants tend to “stick” to soil particles by the process of adsorption. For this reason, even conventional, pressure-driven methods of soil remediation can only remove a fraction of the initial amount of contaminant. Electroosmosis, by operating on a particle level, is able to overcome this limitation to a certain extent. Remediation by electroosmosis is dependent upon the level of adsorption of the contaminant and the nature of the soil itself.
An additional benefit of electroosmosis over conventional methods of soil remediation is the fact that electroosmosis overcomes macropore flow, the tendency of water that is applied to soil faster than it can be absorbed and dispersed to move into and through the soil profile mainly through the macropores, bypassing the micropores. This preferential flow, depending upon the particular soil (sandy soils have more macropores, clayey soils have more micropores), means that conventional methods of soil remediation leave a considerable amount of the contaminant behind after treatment.
For these reasons, a deeper exploration of the relationships that affect the efficacy of electroosmosis are important if we wish to most effectively deal with contamination of soil.
PROCEDURE
Preparation
Soil samples with a wide range of attributes for a basis of comparison and analysis of trends were gathered were oven dried to a constant mass (to assure that all moisture was evaporated in the process). Tannic acid solution was prepared by dissolving tannic acid (VWR Sargent Welch; Buffalo Grove, IL) in distilled water to a concentration of 100 mg/L.
The end contacts of a fluorescent lamp were used to construct the anode and cathode of the test chamber. To prepare the anode, two holes were drilled in a circular metal slug from an electrical box. A rubber washer was placed over the filament wires and the wires were threaded through the holes in the metal slug. The end contact, the rubber washer, and the metal slug were fastened together with epoxy glue. Care was taken to keep the ends of the filament wires and the exposed surface of the metal slug free of glue. The filament wires were soldered to the exposed surface of the slug. The end contact of the fluorescent lamp was fastened in the end cap of a fluorescent tube protective cover with epoxy glue. This process was repeated to prepare the cathode for the testing chamber. A clear, plastic fluorescent tube protective cover was cut to a length such that distance from contact to contact would be thirty centimeters. One hole was drilled at the center of the tube (138 mm from both ends of the tube), and two more holes were drilled at 76 mm on either side of the first hole, the total length of the tube being 276 mm.
Soil Analysis
Determining Texture
Fifteen milliliters of a soil sample were added to one 50 mL soil separation tube. The tube was tapped firmly on a hard surface to eliminate air spaces. One milliliter of Texture Dispersing Reagent (LaMotte; Chestertown, MD) was added to the tube, and the sample was then diluted to 45 mL with water. The tube was capped and shaken for two minutes, and then allowed to settle for 30 seconds. The solution from the tube was poured into another tube and allowed to settle for 30 minutes. The amount of sediment in the first tube was divided by the initial amount of soil (15 mL) to calculate the percentage of sand in the soil. The amount of sediment in the second tube was then divided by the initial amount of soil (15 mL) to calculate the percentage of silt in the soil. The percentage of clay in the sample was calculated by subtracting the sum of the other two percentages from 100 %. This was done to obtain a more accurate reading of clay percentage than the measurement of the volume of clay, as the colloidal nature of clay causes it to swell in water. This procedure was repeated for each soil sample. To determine the texture grouping, a soil texture triangle was used, although it was the specific fractions (of mineralogical size group) that were used to sort the data.
Determining Percent Organic Matter
A crucible was weighed, a small amount of an oven-dried soil sample was added, and the crucible was weighed again, to obtain the mass of the soil. The crucible and the soil were heated over a Bunsen burner for one hour. After cooling, the crucible and soil were weighed again and the change in mass calculated. This process was repeated until two consecutive equal readings of mass were obtained. In this procedure, the values obtained for organic matter are not precise, but approximate, as all weight loss upon ignition of the soil samples is assumed to be due to loss of organic matter. The following reaction is that which occurs in this process:
C6H12O6 (O.M.) + 6O2 + heat -> 6CO2 + 6 H2O
(Where O.M. is organic matter)
Determining Percent Pore Space
A known volume of water was added to a known volume of oven-dried soil. The total volume was then recorded. From this, the percentage pore space was calculated using the following formula:
(((VW + VS) – VT)/(VS)) * 100
where VW is the volume of the water, VS is the volume of the soil, and VT is the total volume of the combined water and soil
Determining Densities
The bulk density of each soil sample was calculated as the weight of a known volume of the oven-dried sample. The particle density was then calculated by the following formula:
(M)/(SS* V)
where SS is percent particle space, or ((100 % – % pore space)/(100)), M is mass, and V is volume
Testing Samples
An oven-dried soil sample was saturated with the tannic acid solution so that all pore space was occupied by the solution. One end cap of the test chamber was fastened in place with electrical tape and the testing ports were sealed with tape to prevent leakage of soil or water prior to testing. The soil was added to the tube and the other end cap positioned and fastened in place with electrical tape.
An initial sampling of water was taken prior to the application of electricity in order to establish the initial concentration of tannic acid in the event that it was changed by factors influenced by soil variables.
A direct current of eight volts was then applied to the soil in the test chamber across the end contacts. Every two hours for a total of six hours water samples were taken from each of the testing ports by means of pipettes and tested for tannic acid concentration.
Quantitative comparison of tannic acid was made of 0.5 mL samples of water from each testing port (diluted to 5 mL with deionoized water). One drop of TanniVer 3 Tannin-Lignin Reagent (Hach; Loveland, CO) and one milliliter of a sodium carbonate solution (Hach) were added to each of the three test tubes and each tube was gently swirled to thoroughly mix the solution. Color development in each of the tubes was recorded after 25 minutes via a color comparator (Hach).
DISCUSSION
There are many factors that are known to have an important role in determining the efficacy of electrokinetic methods of soil remediation such as electroosmosis. The charge, solubility, and level of adsorption of both the contaminant and the soil are known to have a certain degree of influence on the efficacy of electroosmotic-related mechanisms in effecting removal of contaminants. The main purpose of this research was to establish whether texture plays a role in determining the efficacy of electroosmosis in removing organic, water-soluble contaminants.
The variables involved in this research are soil texture, organic matter, pore space, and particle density. Initially, the change in concentration of the contaminant at testing ports one and three was used to attempt to establish any relationships that might exist. However, an increase in concentration at port three did not consistently correspond to a decrease in concentration at port one. An attempt was then made to determine which sets of data were more consistent, based on the average skewness and standard deviation of the data points for each set of data (the data for port three being one set and the data for port one being the other set). From this, it was determined that the set of data with the most consistency was the set of data for port three, so it was that set of data which was used as a base for a comparison of the relative strengths of relationships established.
In an attempt to establish which variable was the dominant factor in determining the efficacy of electroosmosis in effecting the removal of organic, water-soluble contaminants from soil, each variable was systematically assumed to be the independent variable. Once making this assumption, it was possible to plot the data against percent concentration change. With the data plotted, trend analyses were made based on the graphs, and the existence of a definite relationship was either supported or refuted. This was done for each of the soil variables, and then the correlations for each variable were compared in order to establish which variable could be most strongly related to electroosmotic efficacy. For the purposes of graphing, “electroosmotic efficacy” was determined by the percent change in concentration of the contaminant over the time elapsed in the experiment. The larger the percent change in concentration (in this case, the percent change being an increase), the greater the efficacy of the electroosmotic effect.
All variables showed a tentative relationship to electroosmotic efficacy; however, some of the correlations were more distinct than others. The strength of the correlations was determined based on the r2 value for the relationships, and the nature of the relationships was determined from the Pearson’s correlation coefficient and a graphical analysis of the data. The weakest correlation was found to be between particle density and electroosmotic efficacy (r2 = 0.0246). A correlation between organic matter and electroosmotic efficacy was found to be not much greater (r2 = 0.0311). Pore space was found to be more closely related to electroosmotic efficacy, with an r2 value of 0.0810; the Pearson’s correlation coefficient for this relationship was found to be -0.2846, indicating that increasing pore space might have a negative effect on electroosmotic efficacy (i.e., efficacy might degrease with an increase in pore space). Of all the variables, however, texture showed the greatest correlation to the electroosmotic efficacy with an r2 value of 0.1007. The texture categories used to calculate the correlation were based on the soil groups defined by the textural triangle. On the numerical scale used to determine this correlation, smaller numbers were used to indicate soils with greater portions of sand and lesser portions of silt and clay. The Pearson’s correlation coefficient for this relationship was -0.3174, a value that indicates a decreasing electroosmotic efficacy with increasing textural category number. From this, then, of all variables the greatest basis for a correlation is between soil texture and electroosmotic efficacy, that correlation being of decreasing efficacy corresponding to a decreasing portion of sand and increasing portions of silt and clay.
To substantiate this trend, analyses were made of correlations between individual size fractions and the electroosmotic efficacy (percentage clay, percentage silt, and percentage sand vs. efficacy comprising three separate sets of data). The Pearson’s correlation coefficient for the relationship of clay to efficacy was found to be -0.2219, indicating a decreasing efficacy with increasing clay percentage and supporting the correlation produced using textural categories based on the textural triangle. The Pearson’s correlation coefficient found between silt percentage and efficacy was -0.2027, indicating the same type of relationship and also corroborating the correlation established earlier. Finally, the Pearson’s correlation coefficient for the relationship of sand to efficacy was 0.2811, indicating increasing efficacy with increasing sand percentage, helping to further validate the initial textural relationship established. Pure mathematical percentages were not used as the primary method of comparing the efficacy of electroosmosis since it is the ratios of sand, silt, and clay that have the greatest impact on the properties of a soil.
The relationship of texture to electroosmotic efficacy established seems contradictory to the relationship established by theory of increasing efficacy to increasing, rather than decreasing, clay percentage. There are several possibilities why the data seem to indicate a relationship contradictory to theory. One possibility is that the relationship of texture to the efficacy of electroosmosis is closer to a parabolic relationship than a linear relationship; if this were the case, electroosmotic efficacy might increase approaching the “extremes” of the soil textural triangle (as clay, silt, or sand percentage approaches 100%).
Another possibility is that the time that was allowed in this research for the movement of the contaminant was not enough to compensate for differing rates and duration of electroosmosis among soil samples. It is possible that a soil with a high electroosmotic rate initially might only maintain that high rate of contaminant movement for a short amount of time, while another soil with a lower electroosmotic rate might be able to maintain that rate for a longer amount of time, resulting in a higher overall efficacy.
The purpose of this investigation was to establish whether or not texture does play a role in determining electroosmotic efficacy, and in that regard, this research has accomplished its goal. It is significant to note that the data produced by this research do support the existence of a correlation between texture and electroosmotic efficacy. Although the data contradict rather than support the relationship of texture to the efficacy of electroosmosis and are therefore not definitive in what they reveal, it is notable that the data do support a correlation which warrants further investigation.
In investigating the potential of electroosmosis as a valuable method of in-situ soil remediation, several factors should be considered in addition to the efficacy of electroosmosis. An investigation of the efficacy of electroosmosis versus conventional methods of soil remediation might help to determine the most applicable texture range of electroosmosis. Further tests including a wider array of soil samples might help to pinpoint a more precise relationship.
Additionally, as it is the goal of any method of soil remediation to cleanse the soil with the minimal amount of disturbance possible, another subject of future investigation should involve determining the effect of electroosmosis not only on the contaminant in the soil, but on other facets of the soil profile and environment. A study of how electroosmosis affects nutrients and how well various plants retain nutrients in their roots under the influence of electroosmosis could become important in determining if a contamination site is a likely candidate for the use of electroosmosis or not. Another aspect which may be important in determining the method of soil remediation in any particular instance is the fauna inhabiting the soil profile and how electroosmosis influences them. One valuable investigation of this subject might attempt to determine whether the electrical current used for electroosmosis interferes with electrical impulses in the bodies of simple organisms such as earthworms. All of these factors have the potential to influence a decision made as to the most effective method of soil remediation, and need to be seriously considered as such.
CONCLUSION
In conclusion, there appears to be a correlation between texture and the efficacy of electroosmosis in effecting the removal of organic, water-soluble contaminants from soil. In order to more clearly establish a trend, however, it is necessary to collect more data in order to smooth the curves of the data. Another trend which became made apparent through the data was that as pore space increases, electroosmotic efficacy decreases. It is significant that this trend is partially independent of texture.
Awards won by this project
Reserve Champion at Lancaster Science and Engineering Fair (March 1998)
Intel Science Talent Search Semifinalist (formerly Westinghouse STS)